Heligmosomoides polygyrus is a trichostrongylid nematode found in small rodents. It's life cycle is direct and involves both free-living and parasitic stages.
Larvae
Eggs contain fully developed larvae 28 hours after being laid. The larvae can be observed moving vigorously within the egg shell. The eggs hatch after 36-37 hours giving rise to to first stage larvae. 28-29 hours after hatching, the L1 larvae molt to give rise to the L2 larval stage. A partial molt occurs 17-20 hours later giving rise to ensheathed L3 infective stage larvae. The L3 are active but non-feeding.
Parasitic stages
Within 24 hrs of infection by gavage, no larvae could be found in the intestinal lumen indicating that the larvae had shed their sheath and penetrated the intestinal mucosa.
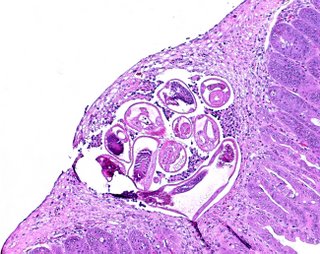
The fourth larval molt takes place about 90-96 hrs after infection. The next molt takes place about 144-166 hrs after infection. By 191 hours most of the worms have passed from the mucosa into the intestinal lumen.

Worms were observed in copula and the first eggs detected in the host feces by 240 hours of infection. The life cycle, from egg to egg, takes about 325 hrs or 13.5 days.
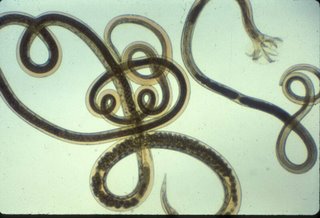
Male and female adult worms. Copulatory bursa can be seen at the posterior end of the male adult worm (smaller worm to the right). The posterior end of the female adult worm is similar to that of an L4. Eggs can be seen in the posterior third of the female adult (larger worm to the left).
Prior descriptions of the Heligmosomoides polygyrus life cycle were described by M.A.M. Famy, 1956, Z. ParasitKde; F.A. Ehrenford, 1954, J. Parasitol. and G.M. Spurlock, 1943, J. Parasitol.
Remember, Heligmosomoides polygyrus was known as Nematospiroides dubius in the early literature.